Who manufactures the optease inferior vena cava (IVC) filter?
Aug 22, 2016 · In 2021, with courts recovering from COVID, the IVC filter lawsuits are heating up. There have been three plaintiffs' verdicts at trial in 2021. The most recent was a $3.3 million verdict in Wisconsin for a woman with a Bard Meridian IVC Filter. Her IVC filter became embedded in her vein.
Do you need an IVC filter lawyer?
Mar 06, 2020 · Understanding the IVC filter injury claim will start with learning the nature of your damages. This means that you and your IVC filter injury attorney should learn how large of a compensation claim you can get for your damages. The United States Food & Drug Administration has published safety communications when it comes to using IVC filters.
What is the Cordis optease IVC filter?
Jun 22, 2018 · 21 Plaintiffs File OptEase® and TrapEase® IVC Filter Lawsuit. The lawsuit was filed by a group of people who were injured by the OptEase® or TrapEase® Vena Cava Filter (“IVC Filter”) manufactured by Cordis Corporation. The first plaintiff, Eleanor C., is a woman from Georgia who was implanted with an OptEase® IVC Filter on August 13, 2009.
What is the difference between the different types of IVC filters?
Feb 01, 2022 · These filters were attractive for patients needing temporary IVC filter therapy. The same cast of characters we have today jumped into the retrievable IVC filters fray: Cook Medical (Gunther-Tulip), Bard Medical (Bard Recovery Filter), and Cordis (Optease). Deeper into IVC Filter science and how it relates to this litigation

What is the average settlement for IVC filter?
between $100,000 and $500,000Based on the IVC filter verdicts and the history of mass tort litigation, settlements may average between $100,000 and $500,000 for significant injury cases but there will certainly be cases that settle higher and lower than that payout range.
What's going on with the IVC filter lawsuit?
Lawsuits filed in federal court allege that defects in the design of IVC filters manufactured by Bard and Cook make them more likely fracture, migrate, tilt or perforate the inferior vena cava. In some cases, when the devices break, pieces can travel through the body, damaging the heart, lungs and other organs.
What type of doctor removes an IVC filter?
Retrievable IVC filter should be removed according to manufacturer and clinical guidelines and assessment. These devices are placed and removed by an IR physician using precision image guidance. The removal procedure is done, usually on an outpatient basis, under light sedation. The process is similar to insertion.
What are symptoms of IVC filter problems?
Inferior vena cava (IVC) filters can cause symptoms ranging from redness and fever to deep vein thrombosis (DVT), pulmonary embolism, and infection....ThrombosisLeg pain.Swelling of the legs.Cramping or soreness in the calf or elsewhere in the leg.The leg might feel warm to touch.Discolored or red skin on the leg.Dec 27, 2019
Can you get an MRI if you have an IVC filter?
You can still have a magnetic resonance imaging (MRI) while you have an IVC filter, but it's important to tell the healthcare provider at the radiology facility each time to be safe.Jan 21, 2021
Can a IVC filter cause shortness of breath?
An IVC filter is a small metal device that traps large clot fragments and prevents them from traveling through the vena cava vein to the heart and lungs, where they could cause severe complications such as pain, difficulty breathing, shortness of breath or even death.
When should an IVC filter be removed?
According to the FDA and Radiological Society of North America IVC filters should be removed once the danger of a life-threatening clot is over. Although the procedure to remove the filter is also minimally-invasive, removing the device may be challenging especially after prolonged dwell times.
Is IVC filter placement a major surgery?
About the Procedure IVC Filter placement and removal is a minimally invasive surgery. The implantation of the IVC filter involves a local anesthetic and numbing medication injected in your skin in the area that the IVC filter will be inserted, preventing discomfort during the surgery.
When should IVC be removed?
A retrievable IVC filter may be removed when the risk of a blood clot traveling to the lungs has passed or if you can take blood thinners. Your doctor may recommend removing the filter when it's no longer needed. IVC retrieval helps reduce the risks of having an IVC filter in your body.Jan 15, 2022
Can you get PE with IVC filter?
For permanent filters, research has shown that breakthrough PE—despite the IVC filter—occurred in 0% to 6.2% of cases. One randomized controlled trial 10 showed that PE occurred in 15.1% of high-risk patients who did not receive IVC filters.
Do patients with IVC filters need anticoagulation?
Conclusion: Inferior vena cava filters can be placed in patients who cannot receive concomitant anticoagulation without placing them at significantly higher risk of development of venous thromboembolism.
Can IVC filters cause strokes?
In the most dangerous and serious instances, an IVC filter can cause death, paralysis, bleedings, stroke, or heart attack.Feb 27, 2018
What happens if your IVC filter gets clogged?
When several clots become trapped in the filter, clot formation down the IVC can cause painful debilitating swelling in the legs. Other signs and symptoms of a problem might include darkening of the skin and ulceration in the lower extremities. Patients may have difficulty identifying the cause of their leg pain.
What are complications of vena cava filter?
What are the risks of an inferior vena cava filter placement?Infection.Excess bleeding.Allergic reaction.Damage to the blood vessel at the insertion site.Blockage of blood flow through the vena cava, which can cause leg swelling.A filter that travels to the heart or lungs, causing injury or death.More items...
How long should IVC filters stay in?
The U.S. Food and Drug Administration (FDA) recommends removing temporary IVC filters after 29-54 days. While this is not very long, it should provide enough time for the acute threat to pass or to find another solution that can work on a long-term basis.Dec 27, 2019
What happens if you leave an IVC filter in too long?
But blood clot filters can also be dangerous. An IVC filter left in too long can perforate the vein or detach from the vein and migrate elsewhere, causing unintended blockages or damage.Dec 15, 2015
Can you get a PE with an IVC filter?
For permanent filters, research has shown that breakthrough PE—despite the IVC filter—occurred in 0% to 6.2% of cases. One randomized controlled trial 10 showed that PE occurred in 15.1% of high-risk patients who did not receive IVC filters.
Can a IVC filter cause shortness of breath?
An IVC filter is a small metal device that traps large clot fragments and prevents them from traveling through the vena cava vein to the heart and lungs, where they could cause severe complications such as pain, difficulty breathing, shortness of breath or even death.
Do patients with IVC filters need anticoagulation?
Conclusion: Inferior vena cava filters can be placed in patients who cannot receive concomitant anticoagulation without placing them at significantly higher risk of development of venous thromboembolism.
Is IVC filter placement a major surgery?
About the Procedure IVC Filter placement and removal is a minimally invasive surgery. The implantation of the IVC filter involves a local anesthetic and numbing medication injected in your skin in the area that the IVC filter will be inserted, preventing discomfort during the surgery.
Is IVC filter placement considered surgery?
An IVC filter is a small, cone-shaped medical device that is placed into your IVC just below your kidneys to prevent blood clots in your legs from traveling to your heart and lungs. IVC filter insertion is a minimally invasive procedure that can be performed on an outpatient basis.
What is the average settlement for IVC filter?
between $100,000 and $500,000Based on the IVC filter verdicts and the history of mass tort litigation, settlements may average between $100,000 and $500,000 for significant injury cases but there will certainly be cases that settle higher and lower than that payout range.
Can you get MRI with IVC filter?
You can still have a magnetic resonance imaging (MRI) while you have an IVC filter, but it's important to tell the healthcare provider at the radiology facility each time to be safe.Jan 21, 2021
WHO removes IVC filters?
Retrievable IVC filter should be removed according to manufacturer and clinical guidelines and assessment. These devices are placed and removed by an IR physician using precision image guidance. The removal procedure is done, usually on an outpatient basis, under light sedation. The process is similar to insertion.
Can IVC filter be removal after 10 years?
In many cases, IVC filters left in place too long, over several months to several years, cannot be safely removed using standard methods because they have scarred into the walls of the vein. “Unfortunately, this complication rate can be really high,” Dr. Kuo told Drugwatch.
What if IVC filter Cannot be removed?
Inside the vein, the IVC filter works by allowing blood to flow around a trapped blood clot until the body's natural blood thinner (anticoagulants) break it down. Without the IVC filter in place, a blood clot traveling to the lung could cause a blockage of the pulmonary artery.
What happens when an IVC filter catches a clot?
When an IVC filter has captured a blood clot traveling through the inferior vena cava vein, the filter clogs and creates a host of medical symptoms, including: Swollen legs, Leg pain, and. The feeling of internal pressure in the legs.
Why Were IVC Lawsuits Unsuccessful at First?
For a long time, no vena cava filter lawsuits so far have made it through trial with a successful jury verdict. Yet many plaintiffs' attorneys rema...
How Much Compensation in an IVC Filter Settlement Can You Expect?
The settlement value of an IVC filter lawsuit is going to depend on the severity of the victim's injuries. If there is a global settlement, there w...
What IVC Filters are Recalled?
The FDA has issued a number of recalls and warnings on IVC filters. Two of the recalls were Class I recalls. Class I means there is a reasonable pr...
How Do You Join the IVC Filter Class Action?
The easiest path to join one of the MDL class actions involving the IVC filters is to hire a lawyer who is handling these cases. That attorney will...
What is a perforation filter?
A perforation is when a part of a filter migrates through the wall of the IVC and leaves the IVC. The morbidity and mortality associated with surgical removal of Bard, Cook Medical, Rex Medical, and Greenfield filters are high.
Where do blood clots travel?
These small, cage-like devices are designed to filter or "catch" blood clots that travel from the lower portions of the body to the heart and lungs. Blood clots in the legs or pelvis can occasionally travel to the lungs, where they could cause a pulmonary embolism (PE) or blockage.
Can IVC filters be removed?
However, new filters that have the option to be removed later on or remain permanent have developed. These retrievable filters should be removed as soon as possible after the risk of a clot traveling to the lungs has passed.
When was the IVC filter invented?
The filter was first manufactured in 1979 and has been inserted into over 260,000 patients. Lawsuits are being filed around the country amid reports that too many of these filters cause more harm than good. They are allegedly prone to fracture, tilt, migrate, perforate the IVC walls, and break apart.
Why was Optease recalled?
In March 2013, about 33,000 OptEase filters were recalled because a labeling error might cause it to be implanted backward. If this occurred, there would be nothing to stop the filter from migrating in the bloodstream to a patient’s heart. Click here to read more.
What is an IVC filter?
Cordis Corporation manufactures the OptEase inferior vena cava (IVC) filter. It is a temporary implant designed to be removed when it is no longer necessary. The longer it remains implanted, the higher the risk of complications.
How long after IVC implantation can I remove?
Due to increased risks like filter fracture, migration, and organ damage, the FDA recommends removing IVC filers 29-54 days after implantation. However, studies have found that real-world retrieval rates remain as low as 20%.
What happens if IVC filter fails?
If health complications occur because of a failing IVC filter, you were probably forced to stay at home until you recover. This means you are not attending work, and therefore, you are losing your wages. If this health issue affects your ability to earn a living, that kind of economic harm can affect your damages. You can get compensation for any income you have lost so far because of your health problems. Also, you are entitled to compensation for the income you would have earned in the future if your health was not damaged.
What happens when you file an IVC lawsuit?
In most states, the law expects injury applicants to minimize or “mitigate” the financial impact of the harm.
Why are Cook and Cordis IVC filters dangerous?
These suits claim these filters have a greater risk of perforation, penetration, tilting, fracture, and migration. The victims bringing these lawsuits would concede that these risks are present in all IVC filters. But the key is whether Cook and Cordis were worse.
How to contact Cook and Cordis IVC?
If you were injured from a Cook or Cordis IVC filter, reach out to our IVC filter lawyers today at 800-553-8082 or connect with us online. The call or online case review is free.
Can IVC filters cause death?
IVC filters have been linked to an increased risk of fracture which can cause serious injury or death. Our law firm is focused on Cordis and Cook IVC filter cases.
What is an IVC filter?
These devices essentially resemble the ribs of an umbrella without the cloth stretched between them. The first IVC filter was the Mobin-Udin filter developed in 1967.
How much did the IVC filter case win?
The big news in these IVC filter cases came in April 2018 when the plaintiff scored their first big win. An Arizona jury awarded a Georgia woman $3.6 million, including $2 million in punitive damages. In November 2019, a Pennsylvania jury awarded $34 million to a Georgia woman who was injured by a Rex Medical Option IVC filter.
When was the first IVC filter invented?
The first IVC filter was the Mobin-Udin filter developed in 1967 . It was replaced by the Greenfield filter in 1973. These first-generation filters required surgical access to the femoral vein for placement and the threshold for their implantation was high.
How many Bard IVC lawsuits were filed?
The Bard IVC MDL class action began in August 2015 with 22 lawsuits. By the time the MDL closed on May 31, 2019, 8,000 cases had been filed. How did we get here?
Cordis IVC Filter Lawsuit
In early December of 2018, twenty-seven people filed a lawsuit against the Cordis Corporation, the manufacturers of the OptEase® Vena Cava Filter, also known as an IVC filter. The plaintiffs allege that these defective IVC filters have resulted in life-threatening blood clots and other serious injuries.
IVC Filters
IVC stands for “inferior vena cava,” the body’s largest vein carrying deoxygenated blood from the lower portions of the body to the heart’s right atrium. IVC filters are web-like devices inserted into the inferior vena cava, that are supposed to trap blood clots so they cannot reach the lungs.
High Failure Rates
The lawsuits filed against Cordis are just the tip of the iceberg, because the failure rates of the IVC filters are very high. In a small study, half of the patients with an IVC filter implantation had a fractured device within roughly four years of implantation.
What is an IVC filter?
The majority of IVC filters has similar conical-shapes with legs (“struts”) projecting out from the base (“head”). These implants are indicated as a treatment for blood clots. Models come in both a jugular or femoral version, depending on which location the surgeon is implanting the filter.
Does Zantac have NDMA?
Zantac (and the generic form Ranitidine) have been found to contain NDMA, a powerful carcinogen that causes cancers throughout the body. There are many types of inferior vena cava (IVC) filters, but they all have the same dangerous IVC filter complications in common.
What is inferior vena cava filter?
There are many types of inferior vena cava (IVC) filters, but they all have the same dangerous IVC filter complications in common. The three medical manufacturers facing the greatest number of lawsuits include: Bard Cook Medical Boston Scientific (Greenfield) The majority of IVC filters has similar conical-shapes with legs (“struts”) ...
What is an IVC filter?
Retrievable or optional IVC filters are sometimes referred to as temporary filters, even though they are FDA cleared for permanent placement. Though there is one IVC filter that is specifically designed to be implanted only on a temporary basis and cannot be used permanently. It is the B. Braun Tempofilter™ II. According to the manufacturer, this device is designed to provide effective filtration for up to three months. The Tempofilter™ II is the successor to the Tempofilter™ I. After several instances of filter migration to the right atrium (which was fatal in two cases ), the development of the Tempofilter™ I was suspended. The stability of the filter has been improved in the Tempofilter™ II, but complications have been reported, including the fracture of a filter leg. Fortunately the leg was embedded in the inferior vena cava wall and did not migrate.
How long after IVC filter placement?
A Japanese study of Cordis’ permament TrapEase IVC filter found that patients who received this filter were at “extremely high risk of strut fractures” as early as two to three years after placement of the filter.
Can IVC filters be removed?
Inferior vena cava (IVC) filters fall into two basic categories—permanent IVC filters, which are designed to remain in place indefinitely and retrievable IVC filters, which can be removed as soon as it medically advisable. Retrievable filters in the U.S. are also cleared by the FDA to serve as permanent filters and are often referred to as “optional” filters.
What is an off label medical device?
The term off-label is most often applied to medications, but may also apply to use of a medical device under conditions other than those for which the device has received FDA clearance. Off-label use of IVC filters is common and is believed to represent more than half of all filters implanted in the United States.
Can IVC filters damage the aorta?
Both of these IVC filters have been associated with high rates of perforation of the inferior vena cava by the filter legs. This not only damages the IVC, but can damage organs outside the IVC, including the aorta, intestines, kidneys, pancreas and spinal column.
What are the different types of Bard IVC filters?
The six Bard filters were the Recovery, G2, G2X, G2 Express, Eclipse and Meridian. Filter fracture and limb embolization (the movement of a fractured filter leg through the IVC to another location, such as the heart or lung) were both higher for the Bard filters than any of the other filters investigated. For more information on complications linked to this filter, visit the Bard IVC Filter page.
What causes occlusion of the inferior vena cava?
This can be caused by the filter capturing blood clots or the tendency of the filter to promote the formation of blood clots. Deep vein thrombosis that occurred after the implantation of the filter. Other serious adverse events.
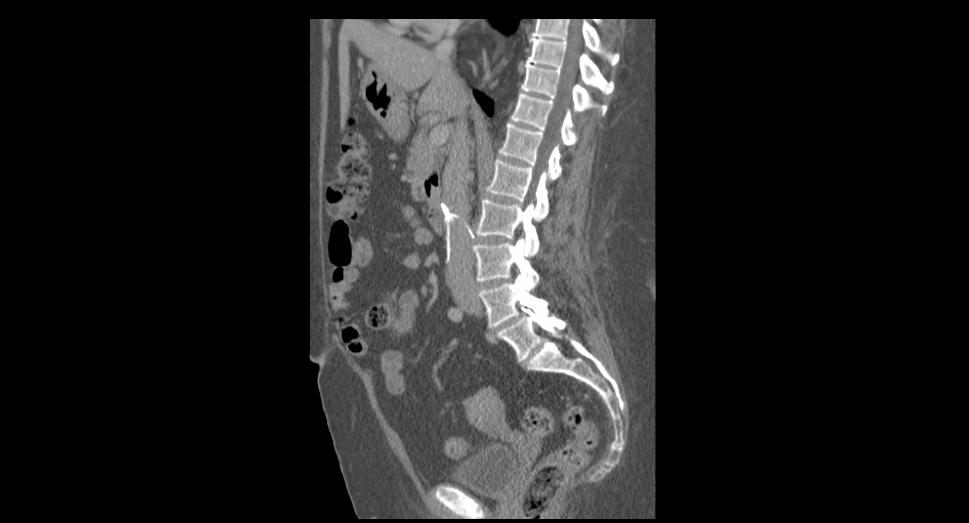
What You Can Do & How We Can Help
- The Schmidt Firm, PLLC is currently evaluating IVC filter cases in all 50 states, regardless of whether you have been injured or not. If you or somebody you know was implanted with an IVC filter, you should contact our lawyers immediately for a free case consultation. Please use the form below to contact our Defective Medical Device Litigation Group or call toll free 24 hours a d…
Overview
- Cordis Corporation manufactures the OptEaseinferior vena cava (IVC) filter. It is a temporary implant designed to be removed when it is no longer necessary. The longer it remains implanted, the higher the risk of complications.
Design Based on Controversial Filters
- A big problem is that its design is closely related to the C.R. Bard Recovery filter, which was withdrawn from the market in 2005 after being linked to a high risk of fracture. However, because the OptEase was approved with a 510(k) application, it has never been studied for safety in clinical trials and the long-term risks are unknown.
Optease Recall
- In March 2013, about 33,000 OptEase filters were recalled because a labeling error might cause it to be implanted backward. If this occurred, there would be nothing to stop the filter from migrating in the bloodstream to a patient’s heart. Click here to read more.
FDA Safety Recommendations
- Due to increased risks like filter fracture, migration, and organ damage, the FDA recommends removing IVC filers 29-54 days after implantation. However, studies have found that real-world retrieval rates remain as low as 20%. The FDA has issued a Safety Communicationto warn about side effects, such as: 1. Filter fracture 2. Migration or tilting 3. Embolization of broken pieces of t…
IVC Filter Lawsuits Centralized in Multi-District Litigation
- Hundreds of people who were seriously injured by side effects of IVC filters have filed lawsuits. Judges have centralized cases against C.R. Bard and Cook Medical in federal courts in Arizona and Indiana. However, this litigation is not a class action — they are individual lawsuits in a Multi-District Litigation (MDL)and each case can have its own outcome.
What Is The Problem?
- The Schmidt Firm, PLLC is nationally recognized as a class action law firm. However, our lawyers are filing individual lawsuits against Cordis Corporationrather than a a class action lawsuit. If you were injured by the OptEase, you could be entitled to compensation.
About Class Actions
- There are many advantages to a class action when a lot of people have similar legal claims. Everyone joins together to collectively seek compensation. Any payout is divided and shared equally.
Why Our Law Firm Is Filing Individual Lawsuits as Opposed to A Class Action
- Class actions are designed for efficiency — not maximizing compensation for individuals with severe injuries from defective medical devices. Members may have to accept “low-ball” settlements or massive attorneys’ fees that leave plaintiffs little to share. Our attorneys file individual lawsuits so we can focus our efforts on helping people with the most serious injuries. …
Do I Have An IVC Filter Lawsuit?
- The Schmidt Firm, PLLC is evaluating IVC filter cases in all 50 states, regardless of whether you were injured or not. If you or someone you know received an IVC filter implant, please contact our lawyers immediately for a free case consultation. You may be entitled to compensation by filing a lawsuit. Please use the form below to contact our Defective Medical Device Litigation Group or c…
Popular Posts:
- 1. "the role of a defense attorney includes all except which one of the following?"
- 2. how binding is medical power of attorney that you sign in the hospital
- 3. how to get a power of attorney without a lawyer free
- 4. what does a patent attorney lawyer do
- 5. why a lawyer would assign a case to a different attorney
- 6. how to find an attorney to look over closing of a home
- 7. attorney general vs supreme court justice who is higher rank
- 8. what happens when attorney does not send signed verification for discovery
- 9. wa state how to be appointed power of attorney when someone dies
- 10. attorney who represents children is called