Under the current rules, § 1305.05(d) requires that a POA be signed by four people: the person who signed the registrant’s most recent application for DEA registration or reregistration, the person to whom the POA is being granted, and two witnesses.
Full Answer
Who is required to execute a power of attorney?
(a) A registrant may authorize one or more individuals, whether or not located at his or her registered location, to issue orders for Schedule I and II controlled substances on the registrant's behalf by executing a power of attorney for each such individual, if the power of attorney is retained in the files, with executed Forms 222 where applicable, for the same period as any …
Can a power of attorney get a DEA form 222?
(d) A power of attorney must be executed by: (1) The registrant, if an individual; a partner of the registrant, if a partnership; or an officer of the registrant, if a corporation, corporate division, association, trust or other entity; (2) The person to whom the power of attorney is being granted; and (3) Two witnesses.
Who can be witnesses to a power of attorney?
(d) A power of attorney must be executed by: (1) The registrant, if an individual; a partner of the registrant, if a partnership; or an officer of the registrant, if a corporation, corporate division, association, trust or other entity; (2) The person to whom the power of attorney is being granted; and (3) Two witnesses.
Who is authorized to sign a power of attorney?
Jan 06, 2022 · The information on this page is current as of Jan 06, 2022. For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 1305.05 Power of attorney....
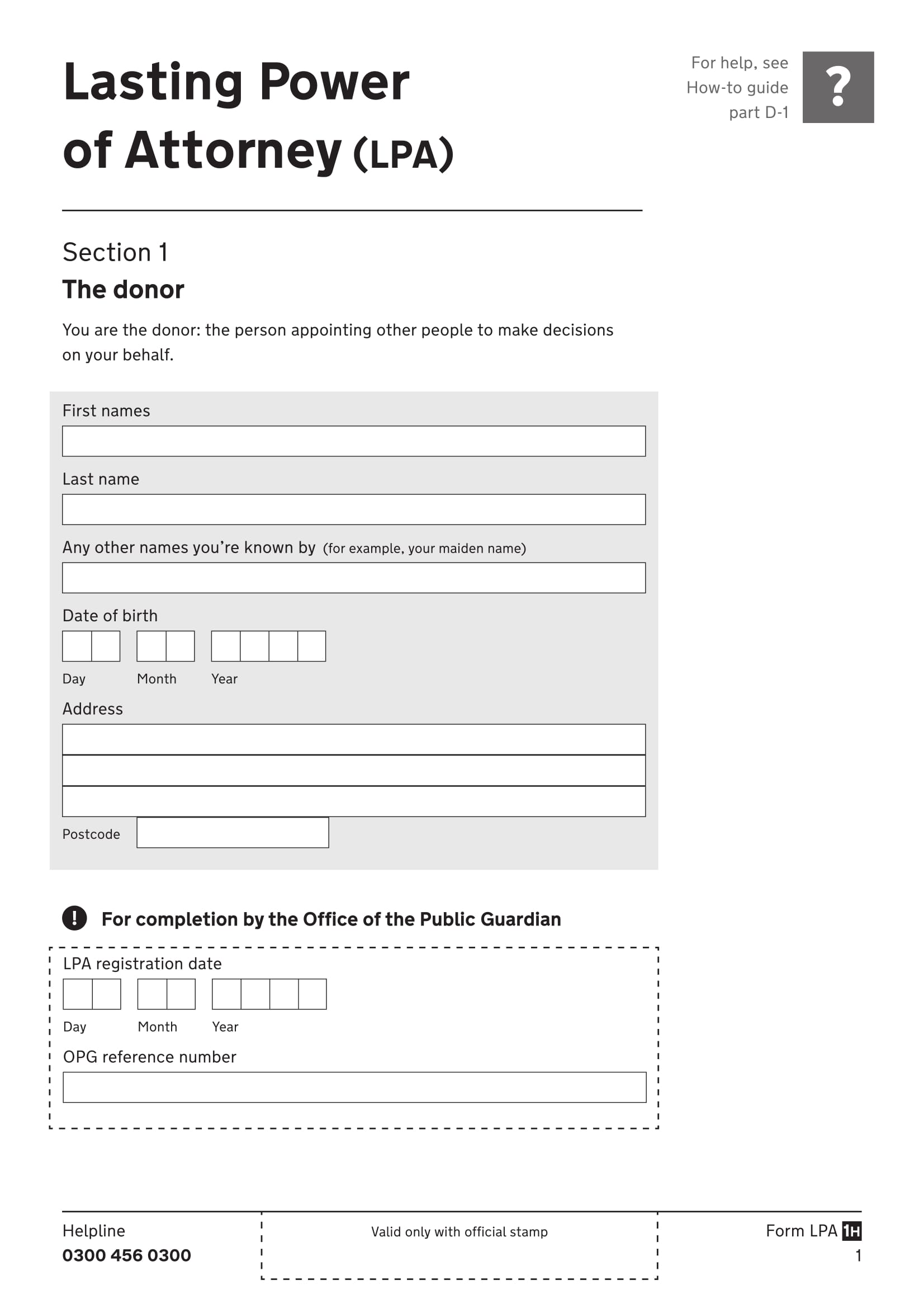
Power of Attorney for DEA Forms 222 and Electronic Orders
I, ____________ (name of person granting power), the undersigned, who am authorized to sign the current application for registration of the above-named registrant under the Controlled Substances Act or Controlled Substances Import and Export Act, have made, constituted, and appointed, and by these presents, do make, constitute, and appoint ____ (name of attorney-in-fact), my true and lawful attorney for me in my name, place, and stead, to execute applications for Forms 222 and to sign orders for Schedule I and II controlled substances, whether these orders be on Form 222 or electronic, in accordance with 21 U.S.C.
Notice of Revocation
The foregoing power of attorney is hereby revoked by the undersigned, who is authorized to sign the current application for registration of the above-named registrant under the Controlled Substances Act or the Controlled Substances Import and Export Act.
How long is a DEA 222 valid?
No DEA Form 222 is valid more than 60 days after its execution by the purchaser, except as specified in paragraph (f) of this section.
When will DEA 222 be accepted?
Triplicate DEA Forms 222 will not be accepted after October 30, 2021. (b) Procedure for executing triplicate DEA Forms 222. (1) A purchaser must prepare and execute a triplicate DEA Form 222 simultaneously by means of interleaved carbon sheets that are part of the triplicate DEA Form 222.
What is a 9333?
9333. (xix) Tincture of opium. 9630. (2) Any salt, compound, derivative, or preparation thereof which is chemically equivalent or identical with any of the substances referred to in paragraph (b) (1) of this section, except that these substances shall not include the isoquinoline alkaloids of opium.
What does "lemaire" mean?
Meaning all parts of the plant presently classified botanically as Lophophora williamsii Lemaire, whether growing or not, the seeds thereof, any extract from any part of such plant, and every compound, manufacture, salts, derivative, mixture, or preparation of such plant, its seeds or extracts.
Can a second supplier fill an order?
The first supplier may not fill any part of an order on an endorsed form. The second supplier may fill the order, if possible and if the supplier desires to do so, in accordance with paragraphs (c) (2) through (4) of this section, including shipping all substances directly to the purchaser.
How to grant a power of attorney?
In order to grant a valid power of attorney, pursuant to 21 CFR 1305.05 (d), the power of attorney must be signed by: 1 The registrant, if an individual; a partner of the registrant, if a partnership; or an officer of the registrant, if a corporation, corporate division, association, trust or other entity; 2 The person to whom the power of attorney is being granted; and 3 Two witnesses.
Is guidance a voluntary document?
To the extent any guidance document sets out voluntary standards (e.g., recommended practices), compliance with those standards is voluntary, and noncompliance will not result in enforcement action. Guidance documents may be rescinded or modified in the Department's complete discretion, consistent with applicable laws.
Is a power of attorney submitted to the DEA?
The power of attorney is not submitted to DEA , but, it must be readily retrievable for inspection. In sum, individuals granted a valid power of attorney under this section may sign DEA 222 order forms. EO-DEA194, October 5, 2020.
Can a DEA sign a 222?
Answer: Registrants, and individuals given power of attorney by registrants, can sign DEA 222 order forms. Any registrant may authorize one or more individuals to obtain and execute DEA Forms 222 by granting a power of attorney to each such individual. 21 CFR 1305.05 (a).

Popular Posts:
- 1. where do i get power attorney
- 2. written memo from assistant district attorney explaining why charges are being broguth up
- 3. how do i register a durable power of attorney for medical
- 4. what is the limits for cja attorney
- 5. what forms are needed to make someone my health care power of attorney nc
- 6. how to use ace attorney investigatol s 2
- 7. what is the average salary for a social security attorney with the federal goverment
- 8. on cross examination what objections can be made to defense attorney
- 9. deputy attorney general california list
- 10. why attorney general jeff sessions survives trump's wrath